Case 31 - In a bit of a Sickle
Author: Dr Angus Perks Reviewer/Editor: Dr Salman Naeem/Nish Cherian/Nick Mani
A 9-year old boy with sickle cell disease presents with a 4-day history of pain in central/left chest with no SOB/cough/fever. SpO2 94% OA, RR 24 with mild increased work of breathing, , HR 92bpm, Temp 37.8.
You are aware of such children are likely to be exposed to significantly more radiation over lifetime, and opt to do a lung ultrasound instead of CXR:-
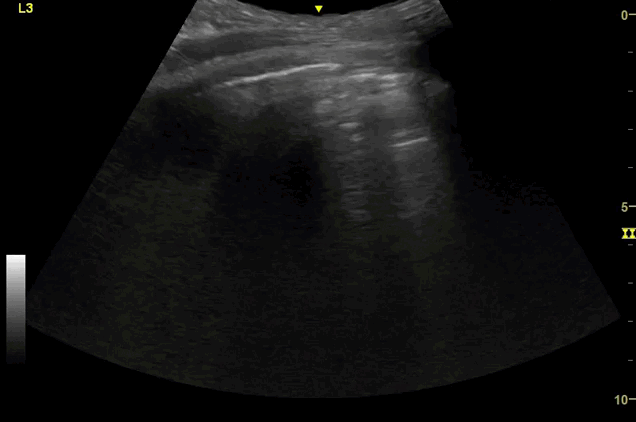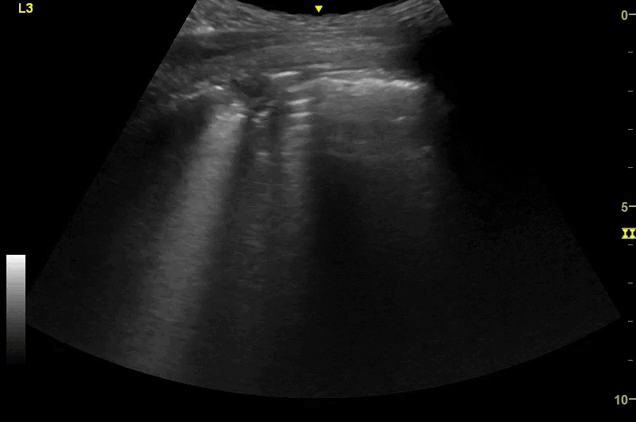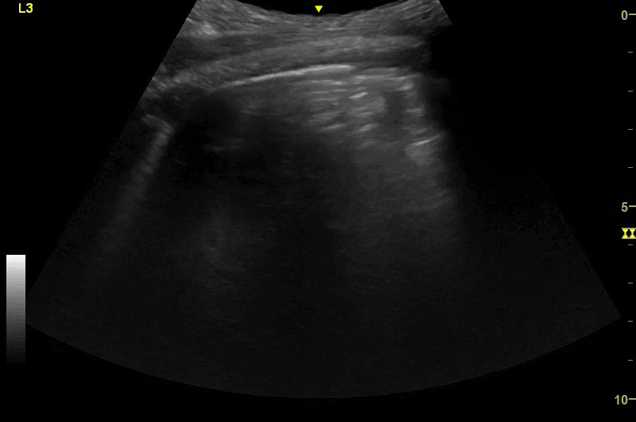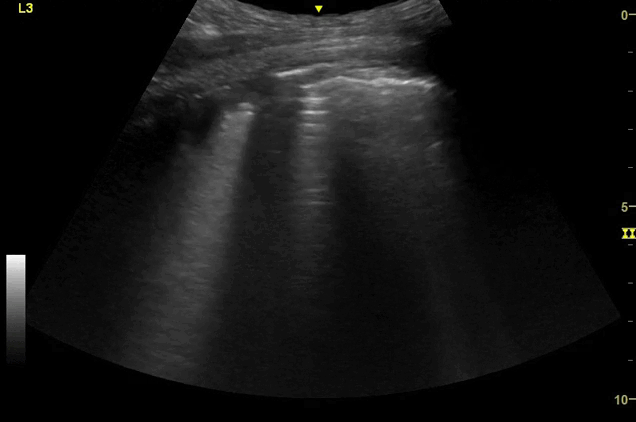
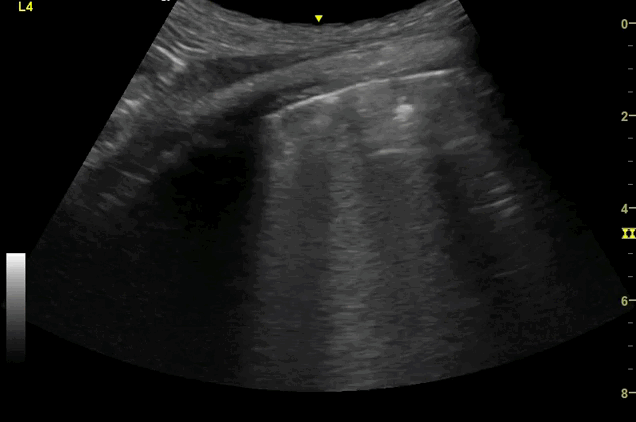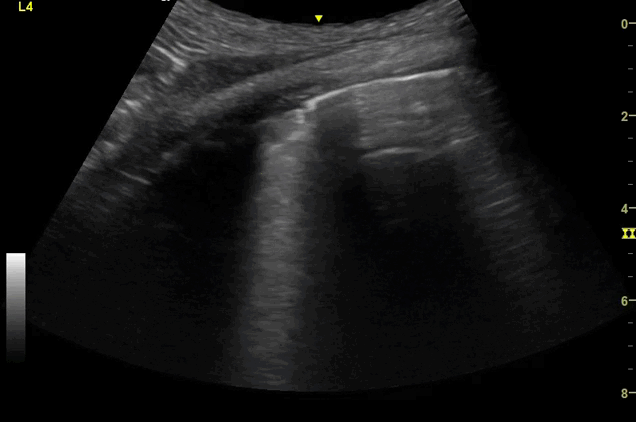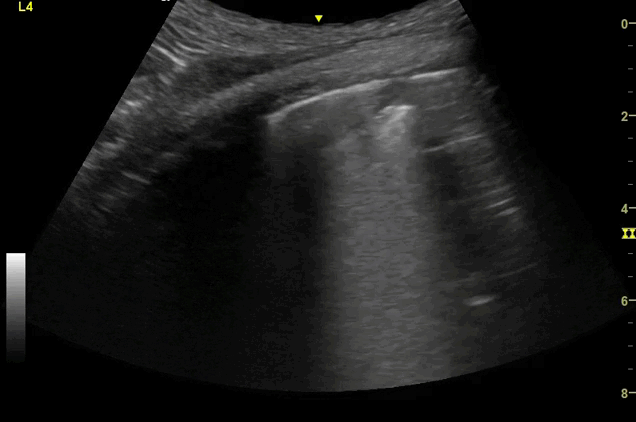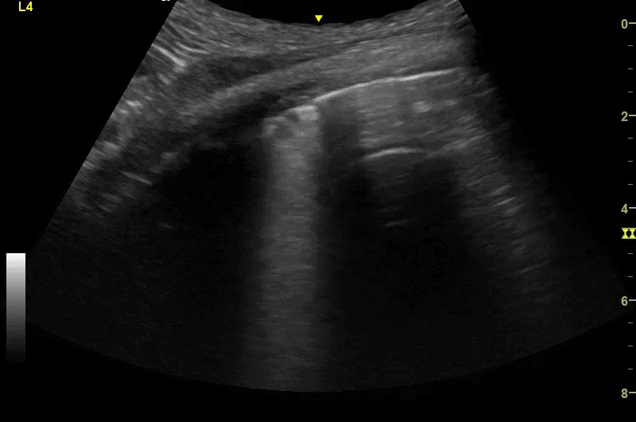
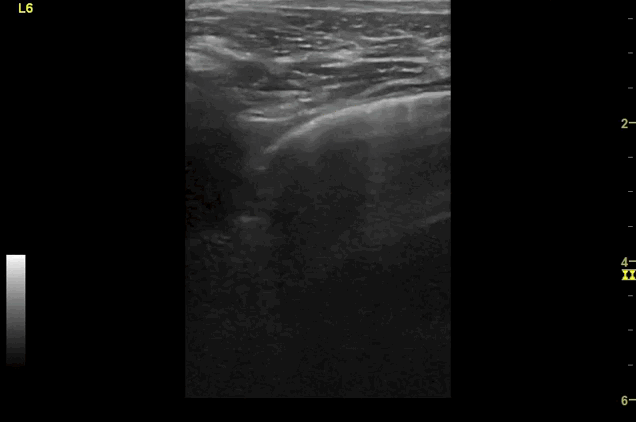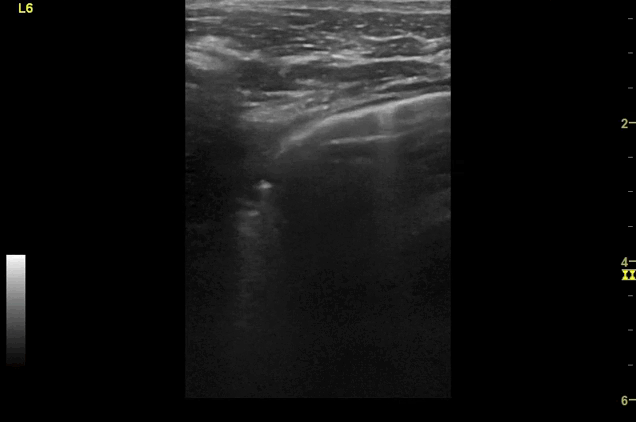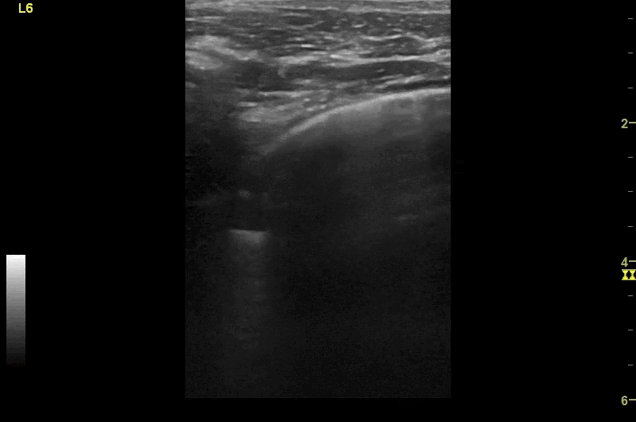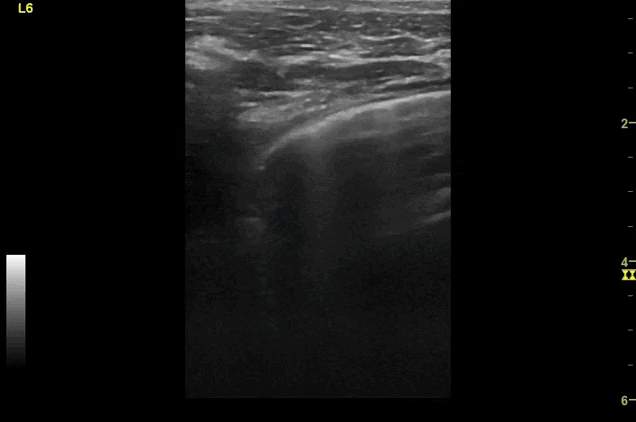
Clips (left to right): Left axilla (L3), left lateral base (L4), left posterior base (L6)
There are a number of different approaches to describe lung ultrasound ‘zones’. These clips use the 12 zones (6 per lung) approach as per the key above and demonstrate views from the left lateral and posterior thorax
-
Areas of pleural irregularity
Sub-pleural consolidation
B-lines, some of which emanate from the sub-pleural consolidation
Adjacent areas of A-line profile (spared areas)
-
‘B-lines’ are well-defined, hyperechoic reverberation artefacts that arise from the pleural line and extend indefinitely. They commonly erase A-lines and move with pleural sliding.[1] They are thought to be due to ring-down artefact caused by air bubbles and sub-pleural thickened interlobular septa, present in alveolar-interstitial syndromes.[1,2]
This can reflect pulmonary oedema (cardiogenic/non-cardiogenic), or other causes of interstitial inflammation or thickening (e.g. pneumonia, interstitial lung disease). There are a number of validated protocols distinguishing pathologies based on the distribution of B-lines and other artefacts such as the BLUE protocol.
In general, if there is a symmetrical, dependent distribution of B-lines without pleural irregularities, this greatly increases the probability of pulmonary oedema and should prompt an echocardiogram to assess for cardiac dysfunction. Indeed, distinguishing exacerbations of COPD vs. LVF is one of the most studied and powerful uses of lung US[3], and is notably more sensitive & specific for pulmonary oedema compared to clinical examination, chest XR and biomarkers (BNP).[4] Additionally, ultrasound is portable and can be used in the prehospital setting.[3]
In this case, alongside the sub-pleural consolidation, patchy A-line profile and irregular pleura, this is very suspicious for infection.
-
In a patient with sickle cell disease, this is Acute Chest Syndrome until proven otherwise.
Acute chest syndrome has a number of causative or contributing factors, including pneumonia (chlamydia, mycoplasma, viral or strep pneumoniae), lung infarction, rib or sternal infarction and fat embolism.[5] Sonographic features include alveolar consolidation, <1cm subpleural consolidations (thought to represent lung infarcts), interstitial syndrome and pleural effusions.
Acute chest syndrome is the leading cause of death in patients with sickle cell disease and so prompt recognition and treatment is essential. Lung ultrasound has been shown to be more sensitive and specific than chest X-ray for the diagnosis of Acute Chest Syndrome[6], and identify abnormalities considerably sooner (3.6 vs. 31.8 hours on average[6]). Patients with SCD and signs of acute chest syndrome will be exposed to a significant amount of ionising radiation over their lifetime, which can be considerably reduced with the adoption of lung ultrasound. This of course requires appropriate governance and documentation of findings.
A common pitfall to be aware of is that the sonographic appearances of the liver, spleen, air in the stomach, and thymus can all be easily mistaken for areas of consolidation and hence false positives[7].
CASE RESOLUTION
The child was treated with antibiotics, analgesia and oxygen and admitted under the paediatric team without needing a CXR. This is in keeping with the ALARA (As Low As Reasonably Achievable) principles.
Take Home Message
Lung ultrasound as an extension of standard assessment could improve decision making, particularly in those that are excessively subjected to radiation such as this case, and recurrent non-traumatic shoulder dislocations (see sound bites 1 & 2).
References

